Timeline
2019
2020
- Langen Junior Science Award
- WHO Declares Pandemic
- Paul Ehrlich and Ludwig Darmstaedter Prize
- Supply Bottleneck for Pneumococcal Vaccines
- Blood Donation Remains Possible
- Convalescent Plasma: First Clinical Trial
- COVID-19 Vaccines: First Clinical Trial
- Marketing Authorisation of Gene Therapy
- COVID-19 Vaccines: Second Clinical Trial Approved
- German Presidency of the EU Council
- COVID-19 Vaccines: Two Clinical Trials Approved
- Another Clinical Trial Approved
- Start of Two Rolling Review Procedures
- Allergen Products: European Standards
- Allergen Product: First European Marketing Authorisation
- COVID-19 Peptide Vaccine: First Clinical Trial
- COVID-19 Vaccine: Third Rolling Review Procedure
- Validation of COVID-19 Rapid Tests
- First Marketing Authorisation of a COVID-19 Vaccine
- SafeVac App Available
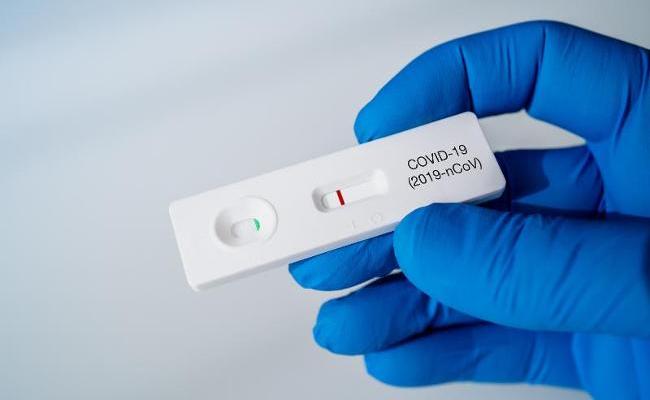
COVID-19 rapid tests are an important pillar in the fight against the pandemic – but they need to be able to detect infections with a high degree of certainty. An independent validation of the tests is not, however, required by law. The testing laboratory for in vitro diagnostic devices at the Paul-Ehrlich-Institut steps into the breach: since September 2020, PEI-IVD has evaluated hundreds of SARS-CoV-2 rapid antigen tests in a standardised study in accordance with the German Coronavirus Test Ordinance (TestV). Since 11 December 2020, the tests that have received a positive assessment have been listed on the Paul-Ehrlich-Institut website.